Abstract
Introduction. Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasm (MPN) that without therapy is a uniformly lethal disease. Historically allogenic stem cell transplant (HCT) was the only curative therapy. In 2001 imatinib was the first CML specific oral tyrosine kinase inhibitor (TKI) approved and since then the treatment of CML has dramatically improved. Subsequently, 2nd and 3rd generation TKIs were developed and have been shown to be superior to imatinib either as initial therapy in certain high-risk patients and in other patients that fail imatinib. However, despite the advances in therapy, patients still die of CML, in fact, in 2020 it is estimated that 8,450 patients will be diagnosed with CML and 1,130 patients will die of CML. HCT remains a curative option for patients with CML. As the safety and availability of HCT improve, we aimed to describe the role of transplant in the modern era of CML therapy.
Methods. We performed a retrospective chart review of patients who underwent their first HCT between June 28, 2006 and December 31, 2021 for CML in either chronic or accelerated phase at the time of transplant.
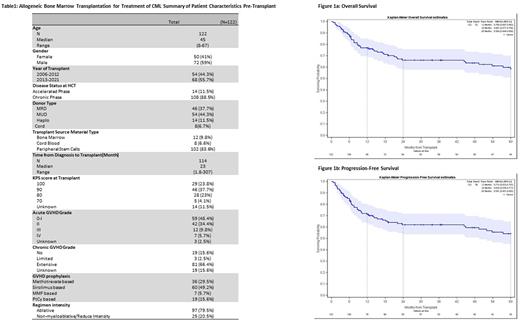
Results. A total of 122 patients were identified. Patient and HCT characteristics are shown in Table 1. The median age at time of transplant was 45 (8-67), 72 (59%) are male. Fifty-four (44.3%) of patients received their transplant between 2006-2012 and 68 (55.7%) of patients received their transplants between 2013-2021. Fourteen (11.5%) patients were in accelerated phase CML at the time of HCT and the rest were in chronic phase. The median time from diagnosis to transplant was 23 months (1.8-307). Ninety-seven patients (79.5%) received ablative conditioning regimens. A total of 46 patients (37.7%) had matched related donors, 54 patients (44.3%) had matched unrelated donors, 14 patients (11.5%) had haploidentical donors, and 8 patients (6.7%) had a cord blood graft. 102 patients (83.6%) received peripheral blood stem cell grafts. GVHD prophylaxis always included a calcineurin inhibitor with the addition of methotrexate in 36 (29.5%) of patients, sirolimus in 60 (49.2%), Mycophenolate in 7 (5.7%), and post-transplant cyclophosphamide in 19 (15.6%) patients. Sixty-one patients (50%) had grade 2 or higher acute GVHD, and 19 (15.6%) had grade 3-4 acute GVHD. Extensive chronic GVHD was present in 81 (66.4%) of patients. Median follow-up of surviving patients was 59.3 months (3.3, 183.8) and overall the median follow-up was 30 months (0.55, 183.8). The estimated 12, 36, and 60-month Overall Survival (OS) of the cohort was 76.9% (95%CI 69.7-85), 66.1% (95% CI 57.7-75.6), and 58.4% (95% CI 49.4-69). Progression free survival (PFS) of the cohort at 12, 36, and 60 months was 71% (95% CI 63.2-79.7), 62% (95% CI 53.6-71.7) and 54.1% (45.1-65%) respectively. The median OS and PFS was not reached (Figure 1a,b). Fifty-two patients in the cohort died, 31 (59.6%) died of complications of treatment, 12 (23.1%) died of progression of disease, and 9 (17.3%) died of unknown causes. The day 100 cumulative incidence of non-relapse mortality (NRM) was 4.1% (1.7-9.7%) and the 1-year NRM was 17.2% (11.5-25.7%).
Conclusion. This study shows excellent long term survival in patients who received an allo HCT for CML. And while, the modern treatment of CML largely relies on oral TKIs, in those patients who do not tolerate TKIs or fail available TKIs, allogeneic transplant remains an active, potentially curable therapy and should be considered.
Disclosures
Koller:Treadwell Therapeutics: Other: Safety Review Committee; Takeda: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Smith:Johnson and Johnson: Current equity holder in publicly-traded company. Ball:Oncovalent: Membership on an entity's Board of Directors or advisory committees. Salhotra:Kadmon: Other: Advisory board meeting ; BMS: Research Funding; Orca Bio: Research Funding. Aribi:SeaGen: Consultancy. Aldoss:Amgen: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Agios: Consultancy, Honoraria; Autolus Limited: Consultancy; AbbVie: Consultancy, Research Funding; Kite: Consultancy. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Stein:Amgen: Speakers Bureau. Artz:Abbvie: Honoraria; Magenta: Honoraria. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings. Nakamura:Magenta Therapeutics: Consultancy; Sanofi: Consultancy; Helocyte Inc: Research Funding; BluebirdBio: Consultancy; Omeros: Consultancy; Kadmon: Consultancy. Al Malki:Gilead: Consultancy, Research Funding; NexImmune: Consultancy, Research Funding; Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Consultancy, Research Funding; CareDx: Consultancy, Research Funding; Incyte: Consultancy, Research Funding. Ali:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal